Sequencing Grade Modified Trypsin
Trypsin Manufactured for Maximum Specificity and Stability
- Lysine residues modified by reductive methylation, yielding a highly active and stable trypsin
- Available lyophilized or frozen
- Referenced in thousands of papers
Catalog Number:
Choose a Format
Size
Catalog Number: V5111
Catalog Number: V5117
Catalog Number: V5113
Trypsin is a serine protease that specifically cleaves at the carboxylic side of lysine and arginine residues. The stringent specificity of trypsin is essential for protein identification. Native trypsin is subject to autolysis, generating pseudotrypsin, which exhibits a broadened specificity including a chymotrypsin-like activity. Such autolysis products, present in a trypsin preparation, would result in additional peptide fragments that could interfere with database analysis of the mass of fragments detected by mass spectrometry. Sequencing Grade Trypsin has been manufactured to provide maximum specificity. Lysine residues in the porcine trypsin have been modified by reductive methylation, yielding a highly active and stable molecule that is extremely resistant to autolytic digestion.
The specificity of the purified trypsin is further improved by TPCK treatment, which inactivates chymotrypsin. The treated trypsin is then purified by affinity chromatography and lyophilized. It is resistant to mild denaturing conditions such as 0.1% SDS, 1M urea or 10% acetonitrile and retains 50% of its activity in 2M guanidine HCl. The activity of trypsin is decreased when acidic residues are present on either side of a susceptible bond. If proline is at the carboxylic side of lysine or arginine, the bond is almost completely resistant to cleavage.
Choose Your Configuration: Learn more about our custom options for this product at: www.promega.com/custom/
Recommended Reaction Buffer: 50mM NH4HCO3 (pH 7.8).
References- Keil-Dlouha, V.V. et al. (1971) FEBS Letters 16, 291–5.
- Rice, R.H. et al. (1977) Biochem. Biophys. Acta 492, 316–21.
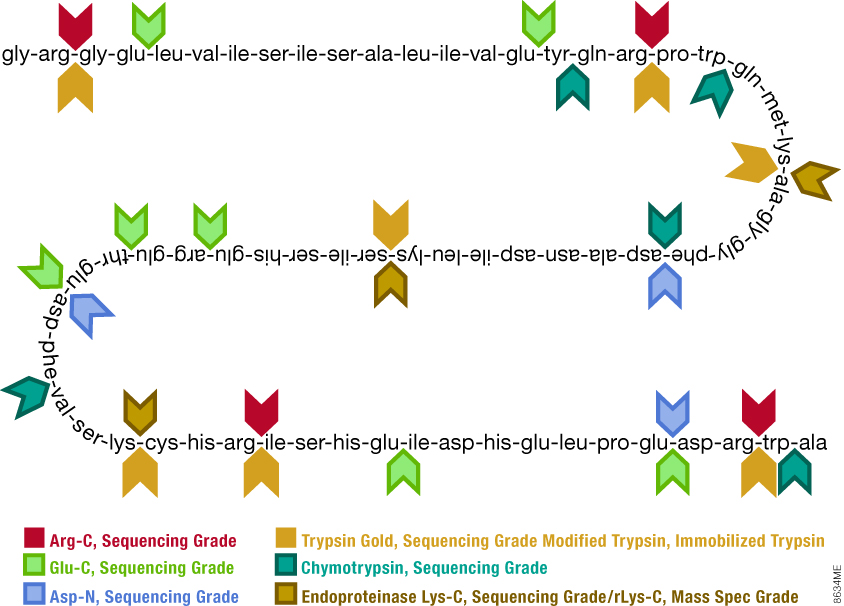
Digest sites for a variety of trypsins and proteases.
Specifications
Catalog Number:
Lieferumfang
| Produkt | Katalognummer | Größe |
|---|---|---|
|
Sequencing Grade Modified Trypsin, Porcine (lyophilized) |
V511A | 5 × 20μg |
|
Trypsin Resuspension Dilution Buffer |
V542A | 1 × 1ml |
SDS
Search for SDSAnalysezertifikat
Nutzungseinschränkung
For Research Use Only. Not for Use in Diagnostic Procedures.Lagerhinweise
Lieferumfang
| Produkt | Katalognummer | Größe |
|---|---|---|
Sequencing Grade Modified Trypsin |
V511B | 1 × 100μg |
|
Trypsin Resuspension Dilution Buffer |
V542A | 1 × 1ml |
SDS
Search for SDSAnalysezertifikat
Nutzungseinschränkung
For Research Use Only. Not for Use in Diagnostic Procedures.Lagerhinweise
Lieferumfang
| Produkt | Katalognummer | Größe |
|---|---|---|
|
Sequencing Grade Modified Trypsin, Porcine, Frozen (liquid 0.5mg/ml) |
V511C | 5 × 20μg |
|
Trypsin Resuspension Dilution Buffer |
V542A | 1 × 1ml |
SDS
Search for SDSAnalysezertifikat
Nutzungseinschränkung
For Research Use Only. Not for Use in Diagnostic Procedures.Lagerhinweise
Related Products
Ähnliche Produkte
Trypsin Platinum, Mass Spectrometry Grade
Maximum digest specificity. Maximum resistance to autoproteolysis.
VA9000
Endoglycosidase H
Characterize protein glycosylation in applications such as monitoring protein trafficking and determining glycan location and structure by mass spectrometry.
V4871, V4875
PNGase F
Catalyzes cleavage of N-linked oligosaccharides between the innermost GlcNAc and asparagine residues of high mannose, hybrid and complex oligosaccharides from N-linked glycoproteins.
V4831
Trypsin/Lys-C Mix, Mass Spec Grade
A mixture of Trypsin Gold, Mass Spec Grade, and rLys-C, Mass Spec Grade, designed to improve digestion of proteins or protein mixtures in solution.
V5071, V5072, V5073
Wird oft zusammen gekauft
Magnetic Proteomics Sample Prep (MPSP) Kit
Magnetic beads for streamlined, SP3-based proteomics sample preparation.
CS3325A04
Arg-C Ultra, Mass Spec Grade
Endopeptidase that cleaves at the C-terminus of arginine residues, including Arg-Pro.
VA1831, VA1832
Lys-C, Mass Spec Grade
Cleavage site: C-terminal of Lys. Optimal pH: 7–9.
VA1170
Chymotrypsin, Sequencing Grade
Serine protease that preferentially hydrolyzes at carboxyl side of amino acids Tyr, Phe and Trp
V1061, V1062
Glu-C, Sequencing Grade
Cleaves at the C-terminus of either aspartic or glutamic acid residues and has optimal activity at pH of 4.0–9.0.
V1651
rLys-C, Mass Spec Grade
Cleaves at the carboxyl side of lysines. Retains proteolytic activity under denaturing conditions such as 8M urea, used with proteolytically resistant proteins.
V1671
IdeS and IdeZ Proteases
IgG-degrading enzymes that cleave at a single site below the hinge region.
V7511, V7515, V8341, V8345